Fieldwork
Gabon 2019
 The mighty Ivindo River, as seen from the Ipassa field station.
The mighty Ivindo River, as seen from the Ipassa field station.
With more than 400 species of brackish and freshwater fish, Gabon abounds in a rich aquatic biodiversity. Among them, are the mormyrids, a group of about 200 species endemic to Africa. One genus Paramormyrops is particularly abundant in Gabon, where approximately 30 species have been recognized. These small fish iemit weak electrical discharges from an electrical organ derived from muscle tissue located in their tail. Mormyrids use these discharges both for communication purposes as well as to form electro-sensory images of their environment and to detect their prey. Often different species are morphologically similar, but emit very distinct electrical discharges from each other, which suggests that these electrical signals play an important role in the recognition of species.
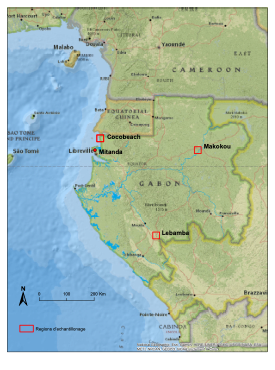
Our Field sites
This study was conducted in three different regions of Gabon:
- 13 days in the costal village of Cocobeach
- 15 days near the southern village of Lebamba
- 8 days of sampling in Ivindo National Park at Ipassa station
- 1 day of sampling at Cap Esterias, near Libreville
Expedition goals
To better understand the diversification of mormyrids, our team worked to collect a variety of genomic, ecological and behavioral data:
1. Study and compare the genetic expression of the electrical organs of different species, to identify the genes that are the basis of the differences in electrical signals (in duration, number of phases, and complexity of the waves emitted). For this theme, we have dissected and preserved the electrical organs of different species of mormyres captured in the three regions visited. We also took genetic samples from most of the individuals collected.
2. Deepen our knowledge of the adaptive function of electrical signals. For this theme, we have concentrated our efforts on a particular species, Paramormyrops kingsleyae, which is the widespread in Gabon. Previous expeditions to Gabon revealed that this species emits a polymorphic electric discharge: in the majority of populations across the country, individuals emit three-phase (triphasic) signals, while in some populations, individuals issue two-phase (biphasic) signals (Picq et al., 2020; Gallant et al. 2011). In two regions of Gabon, Cocobeach and Lebamba, biphasic and triphasic P. kingsleyae are found in very close rivers and even coexist. In order to better understand the forces that generate this intraspecific variation in the electrical signal, we tested two different hypotheses:
 The beautiful Iboundji River, where triphasic P. kingsleyae are found.
The beautiful Iboundji River, where triphasic P. kingsleyae are found.
- First, we tested whether sexual selection could be the basis for the divergence of these signals. This hypothesis predicts that the signals differ between populations and that individuals have a preference for sexual partners emitting the same type of signal as them. We therefore carried out behavioral experiments directly in the field aimed at assessing the preference ofindividuals P. kingselyae between biphasic or triphasic signals.
- Second, we tested whether natural selection could be responsible for this variation. This hypothesis also predicts that the signals differ between populations, and that these different signals make it possible to exploit different ecological resources (prey). To test this, we carried out an inventory of macro-invertebrates in different rivers and collected the stomach contents of species emitting different signals in these rivers to determine if these different signals allow them to detect and ingest different prey.
3. Characterize the internal and external microbial communities of different species of mormyrids. Many animals are colonized by microbial symbionts which have beneficial and fundamentally important effects on the biology of their host. Microbes can regulate animal development, immunity and metabolism, mediate ecological interactions, and facilitate the evolutionary origin and diversification of certain groups. As part of a collaboration with Dr. Benbow of Michigan State University, we also conducted a pilot study aimed at characterizing the microbiomes from mucus samples from the skin as well as from the intestinal wall of different species of mormyridae.
Methods
Capturing Fish
Our main technique for sampling mormyrids consisted of detecting fish using to their electric field using an electrode connected to an amplifier. This non-invasive technique makes it possible to amplify and listen to all the electric fields present in the water and therefore to determine the precise position of the mormyrids without disturbing them. Once the fish were located, the rest of the team caught them using nets.
 Lauren Koenig, MSU Graduate Student, demonstrates this technique. She searches for electric fish with the electrode (here attached to a branch) and listens to them with the amplifier (placed in the blue waterproof bag). Franck Nzigou fishes the fish with the landing net.
Lauren Koenig, MSU Graduate Student, demonstrates this technique. She searches for electric fish with the electrode (here attached to a branch) and listens to them with the amplifier (placed in the blue waterproof bag). Franck Nzigou fishes the fish with the landing net.
In some sites with shallow rocky habitats where the use of landing nets is difficult, we also fished with small hooks attached to fishing line. Even without bait, this technique allowed us to catch a few mormyrids, especially below the Bongolo falls.
In other sites with deeper rock habitats (> 1m), we have also deployed traps inspired by local fishermen. These traps were made of plastic mesh in the shape of a cylinder, with funnel shapes at each end allowing easy entry for the fish but a much more difficult exit. We have threaded earthworms lengthwise on fine straws so that the worms hang in the center of the trap. These traps were deployed at night (because mormyrids are nocturnal) mainly in the Ivindo river, and allowed us to catch many different species of mormyrids.
 Hans Mipounga, IRAF Researcher shows one of the mormyrid traps.
Hans Mipounga, IRAF Researcher shows one of the mormyrid traps.
Capturing Macroinvertebrates
The collection of benthic macro-invertebrates was carried out by taking into account the different aquatic microhabitats (different substrates, types of vegetation) present in each river, paying particular attention to sampling the habitats where the mormyres were present. In each microhabitat, the macroinvertebrates were sampled using a D-frame net with a mesh of 500 μm. For the soil, the sampling technique consisted in driving the net 2 or 3 cm into the substrate, placing the opening of the net in the opposite direction of the water flow (from downstream to upstream) and stir and scrape the bottom substrate with your feet by pushing it towards the inside of the net frame. All of the organic elements (animals and plants) and small minerals are thus washed away at the bottom of the net. Then, the contents of the net are emptied into a container which is brought back to the field laboratory and poured into a series of sieves of three different meshes. To sample the floating plants, the plants are moved using the net and hands, then the net is swept among the plants several times. The contents are also brought back to the field laboratory and poured into the series of sieves.
To sample plant detritus (dead leaves, dead branches), we removed a bundle of branches and dead leaves which we transported to the field laboratory. The leaves and branches were rinsed and meticulously observed one by one to identify the macro-invertebrates. For each sample, the identification of macro-invertebrates was carried out by the team and all the specimens were preserved in alcohol.
 Sampling of macro-invertebrates. A: Sophie Picq and Lauren Koenig collect macro-invertebrates at Bambomo. B: A larva of a chironome. C and D: Sophie Picq, Lauren Koenig and Franck Nzigou identify macro-invertebrates among the detritus.
Sampling of macro-invertebrates. A: Sophie Picq and Lauren Koenig collect macro-invertebrates at Bambomo. B: A larva of a chironome. C and D: Sophie Picq, Lauren Koenig and Franck Nzigou identify macro-invertebrates among the detritus.
The Mobile Laboratory
Once the fish are captured, they were kept alive in coolers with aerators and transported to our field laboratory, where we recorded their electrical signals. The recordings were made in small aquariums containing river water from which the fish came, using electrodes, an amplifier, a digital to analog signal converter, and a computer that runs custom written Matlab software. As these signals are species specific, these recordings are very useful for identifying species.

Hans Mipounga records the electrical signals of the mormyrids
Following EOD recordings, we identified specimens to the species level (where possible) and photographed them in a photo aquarium. Next, we carried out cutaneous mucus sampling using a sterile cotton swab on the chin of the fish because this part is particularly dense in electro-receptors, Finally, we removed several key tissues (electrical organ, stomachs, intestines and gonads) for genetic analysis at our lab in East Lansing, MI.
 The team at work. A and B: Franck Nzigou and Sophie Picq take samples of the bacterial community from the fish. C: Lauren Koenig takes a photo of the fish. D, E, F: Lauren Koenig, Hans Mipounga, Jason Gallant, Sophie Picq, and Nestor Ngoua dissect the fish.
The team at work. A and B: Franck Nzigou and Sophie Picq take samples of the bacterial community from the fish. C: Lauren Koenig takes a photo of the fish. D, E, F: Lauren Koenig, Hans Mipounga, Jason Gallant, Sophie Picq, and Nestor Ngoua dissect the fish.
Behavior Experiments
For the behavioral experiments, the fish were kept alive in coolers with aerators until the night (the experiments were carried out at night (between 6.30 pm and 5 am) because the mormyres are nocturnal). For each experiment, the fish selected for the experiment (“test fish”) was placed beforehand in the experimental cooler for an acclimatization period of 20 minutes. Another fish from the same river (“stimulus fish”) was also placed in another smaller cooler in a PVC tube for the same duration. After the acclimatization period, the experiment began: it consisted in sending two electrical signals simultaneously to the opposite sides of the test fish cooler: a biphasic signal and a three-phase signal. These discharges were emitted at the rate of discharge of the stimulus fish, but we controlled the form of the electrical signal sent through the computer and the Matlab software. In order to be able to analyze the preference of the test fish vis-à-vis the signals sent, this fish was filmed with an infrared camera and its electrical behavior was also recorded with electrodes. Each experiment lasted 35 minutes. The next day, the tested fish were sacrificed by overdosing on clove oil to verify their sex and to preserve a tail fin for further genetic analysis.

Behavioral experiments on P. kingsleyae. A: experimental device. B: The test fish is filmed during the experiment to quantify its interactions with the electrodes emitting different signals. C: Sophie Picq controls the waves emitted during the experiment.
